Which of the following is not a valid resonance form for N2O?
A) 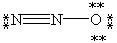
B) 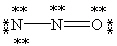
C) 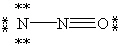
D) 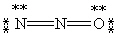
E) All of these are valid resonance forms
Correct Answer:
Verified
Q77: Arrange the following bonds in order of
Q78: Consider the polyatomic ion, (PO3)3 - .
Q79: Arrange the elements K, O, Br in
Q80: Based upon electronegativity differences, which of the
Q81: What is the formal charge of the
Q83: What are the formal charge and the
Q84: Given the table of electronegativity values below,
Q85: Below are two resonance forms of nitrogen
Q86: Which of the following bonds would be
Q87: Which of the following is a valid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents