The molecule PCl2F3 is found to be non-polar . Which shape shown below would be most consistent with this result?
A) 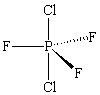
B) 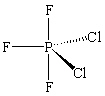
C) 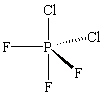
D) 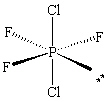
E) 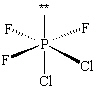
Correct Answer:
Verified
Q94: What type of hybrid orbitals are used
Q95: Which of the following molecule is(are) polar
Q96: What hybrid orbitals are used by iodine
Q97: What hybrid orbitals are used by Bromine
Q98: In which of the following is the
Q100: The fact that the BeF2 molecule is
Q101: The molecule H2CO contains:
(note, consider all the
Q102: What bonded-atom lone-pair arrangement is associated with
Q103: The total number and type of bonds
Q104: The C - C bond in H3C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents