Given the three statements about the molecule HCOOH (correct Lewis structure given below) , pick the best answer. 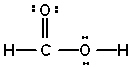 I. HCOOH has 4 s bonds and one p -bond.
I. HCOOH has 4 s bonds and one p -bond.
II. The carbon atom is sp 2 hybridized.
III. The O atom bonded to H atom is sp 3 hybridized.
A) I and II are true, III is false
B) II and III are true, I is false
C) I and III are true, II is false
D) all three are true
E) only II is true
Correct Answer:
Verified
Q104: The C - C bond in H3C
Q105: The C - C sigma bond in
Q106: How many p bonds are present in
Q107: From valence bond theory , what is
Q108: The number of p bonds present in
Q110: Pick the true statement listed below about
Q111: The molecule HCN contains:
(note, consider all the
Q112: How many pi ( p ) bonds
Q113: What is the bond order for the
Q114: What is the bond order for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents