Exhibit 11-2 The phase diagram below is needed for the following question(s) . 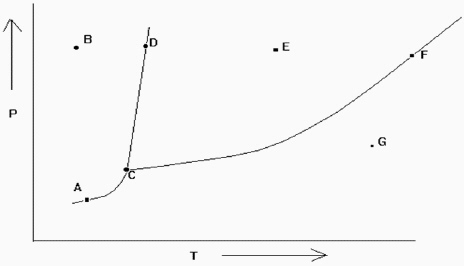
Refer to Exhibit 11-2. If the temperature and pressure are such that the substance is at point D in the diagram, a slight increase in temperature would cause what type of change?
A) change a solid into a liquid
B) change a liquid into a solid
C) go from a solid-liquid equilibrium to a liquid
D) go from a solid-liquid equilibrium to a solid
E) go from a liquid-gas equilibrium to a gas
Correct Answer:
Verified
Q19: Which of the three states of matter
Q20: The temperature at which solid is in
Q21: Exhibit 11-2 The phase diagram below is
Q22: Exhibit 11-2 The phase diagram below is
Q23: What intermolecular force(s) of interaction is(are) possible
Q25: What intermolecular force(s) of attraction is(are) present
Q26: What intermolecular force(s) of interaction is(are) present
Q27: Formaldehyde is a colorless gas but when
Q28: What intermolecular force(s) of attraction is(are) present
Q29: Exhibit 11-3 Consider the General Phase Diagram
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents