Which of the following molecules cannot form hydrogen bonds as a pure liquid?
A) H - F
B) 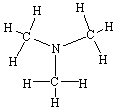
C) 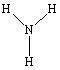
D) 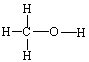
E) All of these can hydrogen bond
Correct Answer:
Verified
Q50: Based upon intermolecular forces of attraction, which
Q51: Which molecule listed below would have its
Q52: Arrange the following elements in order of
Q53: Which of the following forces between atoms
Q54: Given the three statements below, pick the
Q56: On the basis of intermolecular forces of
Q57: Which of the molecules shown below does
Q58: What is the most important intermolecular force
Q59: Which of the following would have a
Q60: Based upon an analysis of intermolecular forces
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents