Exhibit 11-4 Consider Aluminum metal that crystallizes in a face centered cubic cell to answer the following problem(s) . The atomic radius of an aluminum atom is 1.43 . One face of this unit cell is shown in the diagram below. 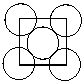
-Refer to Exhibit 11-4. How many aluminum atoms are present in a single face centered unit cell?
A) 1 Al atom
B) 2 Al atoms
C) 4 Al atoms
D) 8 Al atoms
E) 12 Al atoms
Correct Answer:
Verified
Q90: Cesium chloride crystallizes in a unit cell
Q91: Gold crystallizes in a face-centered cubic array
Q92: Calcium fluoride, CaF2, has a unit cell
Q93: A crystal diffracts x-rays (£ = 154
Q94: Exhibit 11-4 Consider Aluminum metal that crystallizes
Q95: A metallic element crystallizes in a face-centered
Q96: A metal that has a radius of
Q97: In a body-centered cubic array of uniform
Q98: Which unit cell(s) provide(s) a coordination number
Q100: At what angle are x-rays with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents