Exhibit 11-4 Consider Aluminum metal that crystallizes in a face centered cubic cell to answer the following problem(s) . The atomic radius of an aluminum atom is 1.43 . One face of this unit cell is shown in the diagram below. 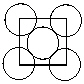
-Refer to Exhibit 11-4. What is the volume of this unit cell?
A) 2.92 Å3
B) 4.84 Å3
C) 23.4 Å3
D) 66.2 Å3
E) 187 Å3
Correct Answer:
Verified
Q76: Iron crystallizes in a body-centered cubic array
Q77: Cohesive forces of attraction are intermolecular attractions:
A)
Q78: The term that best explains why water
Q79: In a cubic array of atoms, how
Q80: A copper crystal is classified as:
A) ionic
B)
Q82: What type of crystal forces hold the
Q83: What type of crystal forces hold the
Q84: The edge of the face-centered cubic unit
Q85: A crystal diffracts x-rays (£ = 154
Q86: A metal that crystallizes in a face-centered
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents