After carefully noting the orientation in which water surrounds each solute particle, which choice below best represents the true nature of how CaCl2 exists in aqueous solution?
A) 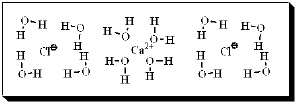
B) 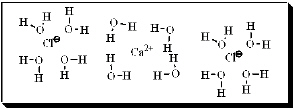
C) 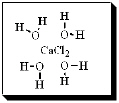
D) 
E) 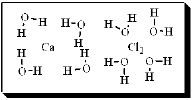
Correct Answer:
Verified
Q86: What is the strongest type of intermolecular
Q87: Which substance listed below would be most
Q88: After carefully noting the orientation in which
Q89: Which set(s) of materials listed below will
Q90: What type of solute-solvent interaction is most
Q92: Which of the following would be least
Q93: Which of the following is soluble in
Q94: Generally a process is considered favorably spontaneous
Q95: Which of the following would be expected
Q96: An aqueous solution of Ag2SO4 contains 0.28
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents