Consider the following first order reaction for the decomposition of dinitrogen pentoxide:
N₂O5(g) 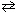 2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
A) 6.25×10 - 3 sec
B) 58.3 sec
C) 102 sec
D) 134 sec
E) 177 sec
Correct Answer:
Verified
Q99: What is a half-life ?
A) It is
Q100: A reaction in solution, A + B→P,
Q101: For the second order rate law,
Q102: At 1000 ° C, cyclobutane (C4H8) decomposes
Q103: Exhibit 13-14 Consider the decomposition of N2O5
Q105: A biochemist studying the breakdown in soil
Q106: Consider the following reaction that follows a
Q107: Exhibit 13-15 Consider the decomposition of NO2
Q108: Exhibit 13-16 Consider the reaction below, which
Q109: At 25 ° C, hydrogen iodide decomposes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents