At 500 C, K p = 4.9*10- 5 for the exothermic reaction:
N₂(g) + 3 H₂ (g) 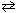 2 NH₃(g) The value of K p at 1000 C is:
2 NH₃(g) The value of K p at 1000 C is:
A) greater than 4.9×10 - 5
B) less than 4.9×10 - 5
C) equal tO4.9×10 - 5
D) equal to 0.00
E) insufficient information to reach a conclusion
Correct Answer:
Verified
Q64: For the high temperature equilibrium system, 2
Q65: Which system at equilibrium shifts right upon
Q66: Consider the following endothermic reaction at equilibrium:
2
Q67: The only factor that causes a change
Q68: Which of the following reactions is product
Q70: What is the effect upon addition of
Q71: Which of the following reaction(s) at equilibrium
Q72: Inert helium gas was injected into a
Q73: Which of the following reactions is product
Q74: The equilibrium governed by the reaction 4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents