Exhibit 14-6 Consider the reaction at equilibrium below and its corresponding equilibrium constant to answer the following question(s) .
2 NO₂ (g) 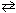 2 NO (g) + O₂(g) K p = 4.48*10- 13
2 NO (g) + O₂(g) K p = 4.48*10- 13
A pressure of 0.45 atm of NO₂ is introduced into a container and allowed to come to equilibrium. Complete the ICE equilibrium table shown below to get started. Partial pressure S2NO₂2 NO O₂Initial 0.45 M Change At equilibrium
-Refer to Exhibit 14-6. What relationship listed below can be used to solve for x in this table and ultimately the equilibrium partial pressures of each substance?
A) x/(0.45 + 2x) = 4.48×10 - 13
B) 2x3/(0.45 - 2x) 2 = 4.48×10 - 13
C) 4x2/(0.45 - 2x) 2 = 4.48×10 - 13
D) 4x3/(0.45 - 2x) 2 = 4.48×10 - 13
E) 4x3/(0.45 + 2x) = 4.48×10 - 13
Correct Answer:
Verified
Q122: What is the equilibrium constant expression for
Q123: What is the equilibrium constant expression for
Q124: What would be the equilibrium constant expression
Q125: At 1073 K, the pressure of CO₂
Q126: For the reaction NH ₄NO₂ (s)
Q128: Exhibit 14-6 Consider the reaction at equilibrium
Q129: Exhibit 14-6 Consider the reaction at equilibrium
Q130: Exhibit 14-5 Consider the decomposition reaction of
Q131: Exhibit 14-5 Consider the decomposition reaction of
Q132: What is the equilibrium constant expression for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents