In the unusual acid-base reaction HClO₄ + H₂ SO₄ 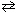 ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
A) HClO4, H2SO4
B) H2SO4, ClO4 -
C) ClO4 - , H3SO4+
D) H3SO4+, H2SO4
E) HClO4, H3SO4+
Correct Answer:
Verified
Q2: What is the conjugate acid of the
Q3: For the reaction CN - (aq) +
Q4: Which of the following is a property
Q5: Consider the following Bronsted-Lowery acid-base reaction:
HClO ₂
Q6: What is the conjugate acid of HSO41
Q8: Which aqueous solution(s) below is(are) considered basic
Q9: Which of the following aqueous solutions is(are)
Q10: What is the conjugate acid of HBO32
Q11: Which species listed below is the conjugate
Q12: The conjugate acid of HPO42 -
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents