Exhibit 15-3 Ephedrine (C10H15ON) , a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base :
C10H15ON (aq) + H₂ O 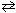 HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
Solution of ephedrine that has a pH of 11.33 to answer the following problem(s) .
-Refer to Exhibit 15-3. What is the base ionization constant , K b , for the dissolution of ephedrine in water?
A) 6.3×10 - 22
B) 7.5×10 - 11
C) 1.3×10 - 4
D) 2.1×10 - 3
E) 6.4×10 - 2
Correct Answer:
Verified
Q115: What is the hydronium ion concentration, [H3O+],
Q116: What is the pH of a 0.25
Q117: Which gives an acidic solution when dissolved
Q118: Which statement below is true regarding the
Q119: Which solution is most basic, given K
Q121: What species are formed in the hydrolysis
Q122: Consider adding NaClO4 to a basic solution.
Q123: K b for ammonia is 1.8×10 -
Q124: Employing a neutralization reaction , which reactants
Q125: A water soluble salt composed of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents