Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 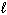 )
) 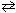 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the base ionization constant , K b , for this reaction?
K a (HF) = 6.8 10 - 4
A) K b = - 6.8×10 - 4
B) K b = 6.8×10 - 18
C) K b = 1.47×10 - 11
D) K b = 1.47×10 - 4
E) K b = 6.8×1010
Correct Answer:
Verified
Q138: Which of these aqueous solutions would have
Q139: What species are formed in the hydrolysis
Q140: What is the base ionization constant ,
Q141: Exhibit 15-5 Consider solving for the pH
Q142: Which aqueous solution listed below would have
Q144: Exhibit 15-6 Consider an aqueous 0.25 M
Q145: What is the pH of a 0.20
Q146: Exhibit 15-4 Consider dissolving NaF in water
Q147: Exhibit 15-5 Consider solving for the pH
Q148: Acetic acid has K a = 1.8×10
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents