Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 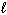 )
) 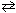 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the pH of this 0.100 M NaF solution?
A) pH = 2.08
B) pH = 5.91
C) pH = 8.08
D) pH = 11.83
E) pH = 11.92
Correct Answer:
Verified
Q153: What is the pH of an aqueous
Q154: An acid HB+ has a K a
Q155: Which of the following substances would be
Q156: Exhibit 15-6 Consider an aqueous 0.25 M
Q157: Exhibit 15-6 Consider an aqueous 0.25 M
Q159: What is the pH of an aqueous
Q160: A 0.050 M solution of ammonium chloride
Q161: Consider the acid strengths of the binary
Q162: Regarding acid strength, which of the following
Q163: Identify the Lewis acid in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents