What volume of 0.200 M H₂ SO₄is required to neutralize 50.0 mL of 0.100 M NaOH?
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 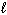 )
)
A) 50.0 mL
B) 25.0 mL
C) 100 mL
D) 12.5 mL
E) 6.25 mL
Correct Answer:
Verified
Q2: In the titration of 0.100 M HCl
Q3: Calculate the pH of a solution made
Q4: Calculate the volume of 0.100 M HCl
Q5: Calculate the pH of a solution after
Q6: A 25.0 mL portion of 0.0500 M
Q8: Solutions are made by combining equal amounts
Q9: What is the molarity of Ba(OH)2 solution
Q10: In the titration of 50.0 mL of
Q11: Which pair(s) of substances would make a
Q12: Calculate the pH of a solution formed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents