A 0.40 gram sample of NaOH (molar mass = 40.0 g/mol) was completely neutralized witH₆2.5 mL of H₂ SO₄solution. Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 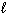 ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:
A) 0.080 M
B) 0.16 M
C) 0.62 M
D) 6.4 M
E) 3.2 M
Correct Answer:
Verified
Q14: During a titration, 60.0 mL of 0.010
Q15: If it takes 32.0 mL of 0.100
Q16: A 350 mL volume of 0.150 M
Q17: What is the pH of a solution
Q18: In the titration of 0.100 M HCl
Q20: What is the volume of 0.0175 M
Q21: Which one of these combinations would give
Q22: Consider a buffer solution containing a weak
Q23: What is the pH of a buffer
Q24: A solution of HCOOH ( K a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents