Consider the ionization of hydrofluoric acid as follows:
HF + H₂ O 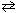 H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF) , to this equilibrium system?
A) The equilibrium shifts to the right and the pH lowers.
B) The equilibrium shifts to the left and the pH lowers.
C) The equilibrium shifts to the right and the pH rises.
D) The equilibrium shifts to the left and the pH rises.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.
Correct Answer:
Verified
Q50: Which of the following titration curves listed
Q51: Consider the ionization of acetic acid as
Q52: What is the pH of a buffer
Q53: What is the pH after the addition
Q54: A buffer is made by dissolving 0.10
Q56: What is the pH of an aqueous
Q57: Exhibit 16-1 Consider the equilibrium reaction below
Q58: What is the pH of an aqueous
Q59: Consider the following equilibrium reaction:
NH₃+ H₂ O
Q60: Calculate the number of moles of sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents