Which of the following titration curves listed below best represents a curve for the complete titration of the weakly basic dianion, sulfide, S2 - , with a strong acid such as HCl as shown below in the net ionic equation?
S2 - (aq) + 2 HCl (aq) →H2S (g) + 2 Cl -
A) 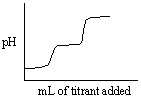
B) 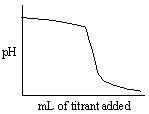
C) 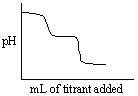
D) 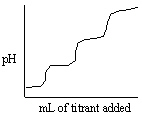
E) 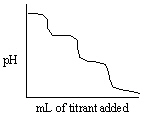
Correct Answer:
Verified
Q57: Exhibit 16-1 Consider the equilibrium reaction below
Q58: What is the pH of an aqueous
Q59: Consider the following equilibrium reaction:
NH₃+ H₂ O
Q60: Calculate the number of moles of sodium
Q61: The titration of a weak acid, HA
Q63: The pH at the equivalence point in
Q64: Exhibit 16-3 Consider the Titration curve below
Q65: Which of the following titrations would produce
Q66: Consider titrating a triprotic acid with standard
Q67: The titration of a weak acid, HA
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents