Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s) . CH ₃COOH + NaOH CH ₃COONa + H₂ O 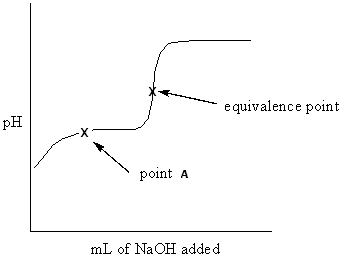
Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
A) This is a problem of solving for the pH of an aqueous weak acid solution.
B) This is a problem of solving for the pH of an aqueous weak base solution.
C) This is a problem of solving for the pH of an aqueous buffer solution.
D) This is a problem of solving for the pH of an aqueous strong base solution.
E) This is a problem of solving for the pH of an aqueous salt solution.
Correct Answer:
Verified
Q69: Consider the titration of a weak acid
Q70: A 20.0 mL sample of lactic acid
Q71: Which of the following titration curves listed
Q72: The titration of a weak base (
Q73: A sample of 0.100 mole of acetic
Q75: Exhibit 16-2 Consider titrating CH3COOH with standard
Q76: What is the pH of solution formed
Q77: What is the pH of the following
Q78: The sharpest inflection point is observed in
Q79: Which experiment listed below would provide a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents