When a 3.125 g sample of ammonium nitrate (NH ₄NO3 , molar mass = 80.05 g/mol) decomposes in a bomb calorimeter with a heat capacity oF4.116 kJ/ C, the temperature rises from 24.15 C to 25.35 C. What is D E for the decomposition of ammonium nitrate?
NH ₄NO3(s) N₂O (g) + 2 H₂ O ( 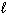 )
)
A) - 126 kJ
B) - 69.5 kJ
C) - 4.94 kJ
D) - 1.58 kJ
E) none of these
Correct Answer:
Verified
Q10: One mole of an ideal gas at
Q11: From the D E given for 25
Q12: Which thermodynamic parameter listed below measures the
Q13: Which substance listed below would be expected
Q14: If only work of expansion is done,
Q16: The combustion of a 0.440 g sample
Q17: Which one of the following systems shows
Q18: If q of the system is negative:
A)
Q19: One mole of a gas, initially at
Q20: Calculate the work done by 1.00 mol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents