The combustion of a 3.06 g sample of formic acid (H CO₂ H( 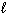 ) , molar mass = 46.03 g/mol) in a bomb calorimeter with a heat capacity of 5.423 J/ C causes the temperature to rise from 23.27 C to 26.41 C. What is D E for the reaction?
) , molar mass = 46.03 g/mol) in a bomb calorimeter with a heat capacity of 5.423 J/ C causes the temperature to rise from 23.27 C to 26.41 C. What is D E for the reaction?
2 H CO₂ H ( 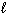 ) + O₂(g) 2 CO₂ (g) + 2 H₂ O (
) + O₂(g) 2 CO₂ (g) + 2 H₂ O ( 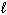 )
)
A) - 5.56 kJ
B) - 17.0 kJ
C) - 256 kJ
D) - 512 kJ
E) none of these
Correct Answer:
Verified
Q1: For which of the following reactions is
Q3: From the D E given for 25
Q4: Which of the following units express(es) a
Q5: When a sample of an ideal gas
Q6: Which of the following statements is incorrect
Q7: Which of the following systems show(s) a
Q8: A sample of an ideal gas at
Q9: The mathematical form of the first law
Q10: One mole of an ideal gas at
Q11: From the D E given for 25
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents