The following reduction of Fe2 O3 to Fe occurs in blast furnaces during the steel making process:
3 CO (g) + Fe2 O3(s) 2 Fe (s) + 3 CO₂ (g) Calculate 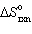 per mole of Fe2 O3 reacted, if the values of S for CO (g) , Fe2 O3(s) , Fe (s) , and CO₂ (g) are 198, 87, 27, and 214 J/mol K, respectively (answer in units of J/K) .
per mole of Fe2 O3 reacted, if the values of S for CO (g) , Fe2 O3(s) , Fe (s) , and CO₂ (g) are 198, 87, 27, and 214 J/mol K, respectively (answer in units of J/K) .
A) - 44 J/K
B) - 15 J/K
C) 15 J/K
D) 44 J/K
E) 411 J/K
Correct Answer:
Verified
Q17: Which one of the following systems shows
Q18: If q of the system is negative:
A)
Q19: One mole of a gas, initially at
Q20: Calculate the work done by 1.00 mol
Q21: Which of the following reactions has the
Q23: The third law of thermodynamics states that:
A)
Q24: For which of the following processes will
Q25: Which of the following physical changes would
Q26: For the reaction 4 CuO (s)→2 Cu2O
Q27: Which of the following chemical equations would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents