Using the entropy values in the table below, what is the change in standard entropy, D S , for the following reaction?
2 CH₃ OH (g) + 3 O₂(g) 2 CO₂ (g) + 4 H₂ O (g) Substance S (J/mol K) CH₃ OH ( 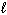 ) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O (
) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O ( 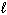 ) 69.91
) 69.91
A) D S ° = - 383.4 J/mol × K
B) D S ° = - 161.8 J/mol × K
C) D S ° = - 40.2 J/mol × K
D) D S ° = 92.3 J/mol × K
E) D S ° = 313.9 J/mol × K
Correct Answer:
Verified
Q26: For the reaction 4 CuO (s)→2 Cu2O
Q27: Which of the following chemical equations would
Q28: Which process below is accompanied by a
Q29: Which of the following reactions would be
Q30: Which of the following physical changes would
Q32: "A spontaneous change is always accompanied by
Q33: Which of the following chemical reactions would
Q34: Which of the following reactions would be
Q35: Which of the following chemical reactions has
Q36: Which of the following processes would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents