Consider splitting water into hydrogen and oxygen gas and its corresponding thermodynamic data as shown below:
2 H₂ O ( 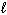 ) 2 H₂ (g) + O₂(g) D S rxn = 326 J/mol K and D H rxn = 572 kJ/mol At what approximate temperature does this reaction cross-over from being considered non-spontaneous to being spontaneous?
) 2 H₂ (g) + O₂(g) D S rxn = 326 J/mol K and D H rxn = 572 kJ/mol At what approximate temperature does this reaction cross-over from being considered non-spontaneous to being spontaneous?
A) It won't cross over. It is spontaneous at all temperatures.
B) It won't cross over. It is non-spontaneous at all temperatures.
C) 186 K
D) 570 K
E) 1.75×103 K
Correct Answer:
Verified
Q42: The reaction PCl3 (g) + Cl2 (g)→PCl5
Q64: Consider the following reaction and its corresponding
Q65: Exhibit 17-5 Consider the reaction of Barium
Q66: Which of the following conditions describe a
Q67: Exhibit 17-4 Consider the reaction of nitrogen
Q70: Exhibit 17-2 Consider the reaction below and
Q71: The reaction of carbon and water to
Q72: A reaction will proceed spontaneously at any
Q73: For the reaction, PCl5 (g) 
Q74: Exhibit 17-2 Consider the reaction below and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents