In the azide ion, which has the Lewis structure shown below, the oxidation state of the central N atom is: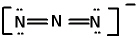
A) +1
B) +1/3
C) - 1/3
D) - 1
E) none of these
Correct Answer:
Verified
Q8: The oxidation state of Cr in Cr2O72
Q11: The oxidation state of S in SO2
Q12: What is the oxidation number of the
Q15: The oxidation state of sulfur in SO3
Q18: The oxidation state of chlorine in ClO4
Q27: What is the oxidation number of the
Q30: What is the oxidation number of the
Q32: What is the oxidation number of the
Q35: The "super-ion battery" contains K2FeO4 as a
Q37: In which of the following compounds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents