What species becomes oxidized and what species behaves as the reducing agent in the following oxidation-reduction reaction?
2 H₂ O ( 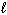 ) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)
) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)
A) Al becomes oxidized and Al behaves as the reducing agent.
B) Al becomes oxidized and MnO41 - behaves as the reducing agent.
C) MnO41 - becomes oxidized and MnO41 - behaves as the reducing agent.
D) MnO41 - becomes oxidized and Al behaves as the reducing agent.
E) MnO41 - becomes oxidized and H2O behaves as the reducing agent.
Correct Answer:
Verified
Q29: In the oxidation-reduction reaction shown below, the
Q30: After balancing the following reaction under acidic
Q31: After balancing the following reaction under basic
Q32: Which equation listed below is correct after
Q33: After balancing the following reaction under basic
Q35: After balancing the following reaction under acidic
Q36: In the following balanced redox reaction:
14 HCl
Q37: Start with the skeleton half-reaction NO3 -
Q38: Given the skeleton half-reaction:
Na2C2O4→CO2 + Na+ When
Q39: Start with the skeleton half-reaction NO2 -
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents