Exhibit 18-1 Consider the figure of a generic voltaic cell below to answer the following question(s) . 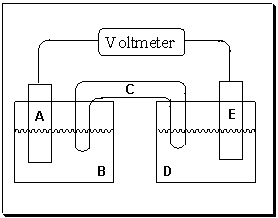
-Refer to Exhibit 18-1. If component A is the positive electrode and component E is the negative electrode, which statement is true?
A) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
B) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
C) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
D) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
E) Electrons are not flowing in this voltaic cell unless it is provided by an outside source.
Correct Answer:
Verified
Q44: A sample containing Fe2O3 is dissolved and
Q45: Exhibit 18-1 Consider the figure of a
Q46: Which of the following half-reactions could occur
Q47: In a voltaic cell:
A) oxidation occurs at
Q48: After balancing the following reaction under acidic
Q50: After balancing the following reaction under basic
Q51: Consider a voltaic cell that operates from
Q52: After balancing the following reaction under acidic
Q53: A 30.0 mL sample of solution containing
Q54: After balancing the following reaction under basic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents