What is the value of the equilibrium constant, K , at 298 K for the following reaction?
Ni (s) + 2 Ag 1+ (aq) 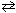 Ni 2+ (aq) + 2 Ag (s) E cell = 1.05 V
Ni 2+ (aq) + 2 Ag (s) E cell = 1.05 V
A) K = 4
B) K = 8×102
C) K = 6×1017
D) K = 3×1035
E) K = 7×1081
Correct Answer:
Verified
Q82: An electrochemical cell that uses a glass
Q83: Consider a voltaic cell made from the
Q84: A concentration cell is built using two
Q85: Calculate Kc for the reaction Br2 +
Q86: Given:
Fe (s) + 2 Ag+ (aq)→Fe2+ (aq)
Q88: Electric current is carried through an aqueous
Q89: Given:
2 U3+ + Cd2+→Cd (s) + 2
Q90: Given:
Zn (s) + Sn2+→Sn (s) + Zn2+
Q91: A voltaic cell is made by combining
Q92: Given the standard reduction potentials:
Cd2+ (aq) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents