When a nuclide with atomic number = Z and mass number = A undergoes 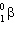 decay, the product nucleus will have:
decay, the product nucleus will have:
A) atomic number = Z, mass number = A
B) atomic number = Z - 2, mass number = A - 4.
C) atomic number = Z + 1, mass number = A.
D) atomic number = Z - 1, mass number = A.
E) none of these
Correct Answer:
Verified
Q1: The transformation of 14C→14N is an example
Q2: The alpha decay of 231Pa produces:
A) "227Ac."
B)
Q3: Which form of radioactive decay can penetrate
Q4: The type of radioactivity that removes excess
Q6: The most common mode of decay for
Q7: The atomic mass of beryllium is 9.012.
Q8: An isotope with Z = 34 is
Q9: 40K undergoes nuclear decay to 40Ar. Which
Q10: Which of the three forms of radiation
Q11: When 31 S undergoes ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents