Multiple Choice
What is the missing particle in the nuclear equation?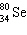 + ____
+ ____ 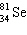 +
+ 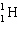
A) 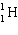
B) 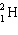
C) 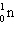
D) 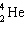
E) none of these
Correct Answer:
Verified
Related Questions
Q61: What is the missing particle in the
Q62: How many neutrons are produced in the
Q63: The mass of an 1H atom is
Q64: In any nuclear decay:
A) The mass of
Q65: What must be placed in the blank
Q67: The mass defect of 31P is 0.2822
Q68: The mass defect of 23Na is 0.2003
Q69: The mass defect of 19F is 0.1587
Q70: What must be placed in the blank
Q71: Curium - 248, 248Cm, was bombarded, yielding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents