A chemist was performing a spectroscopy experiment on a fluorescent dye to examine the influence of concentration on absorbance. To accomplish this, the chemist created a stock solution of a certain concentration of dye using 10 mL of water (H2O) . Then, a serial dilution was performed by taking 1 mL of the stock solution and diluting it with 9 mL of water for a total solution volume of 10 mL. 1 mL was taken from Solution 2 and diluted with 9 mL water to create Solution 3. The serial dilution was performed through Solution 5, as shown in the diagram below. If the dye concentration in Solution 5 was 0.5 g/mL, what was the concentration of the dye in Solution 2?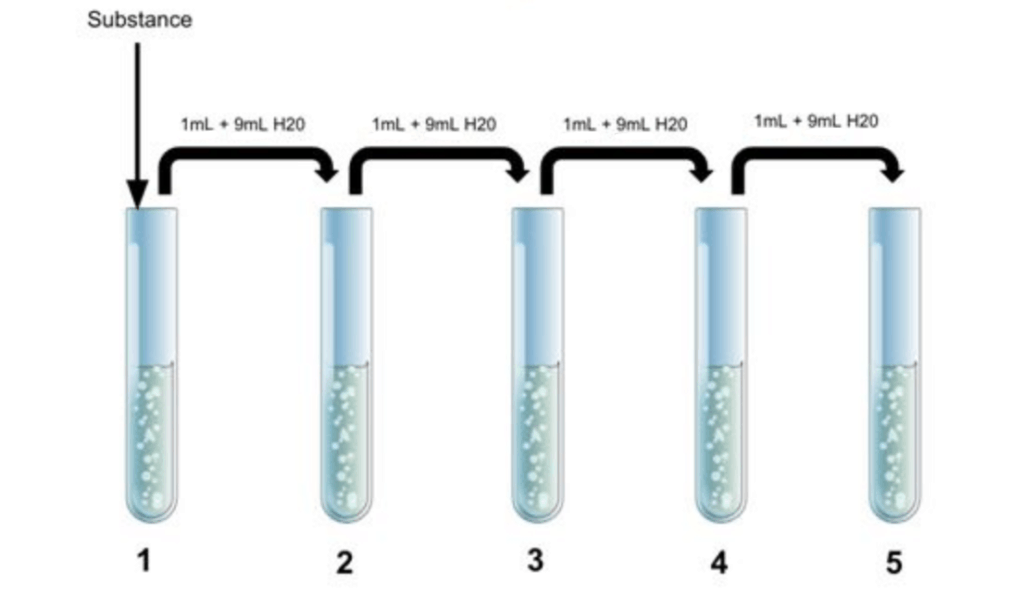
A) 50 g/mL
B) 500 g/mL
C) 5 gmL
D) 5,000 g/mL
Correct Answer:
Verified
Q342: Which would be the approximate mass of
Q343: Which of the following would an electron
Q344: When going to the grocery store, you
Q345: Which of the following is not an
Q346: The International Space Station orbits earth at
Q348: Which of the following would be the
Q349: Which of the following is true about
Q350: Which of the following units is derived
Q351: Which of the following prefixes would likely
Q352: A book weighs 2.2 kg. How much
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents