A large, multinational drug corporation conducts a phase I clinical trial to evaluate the safety profile and pharmacokinetic properties of a new drug designed to treat refractory epilepsy. Initial studies in animals showed that the drug undergoes extensive metabolism by the liver into glucuronidation byproducts that are primarily excreted by the kidneys. The curve below demonstrates the glucuronidation rate of the drug over a wide range of doses. 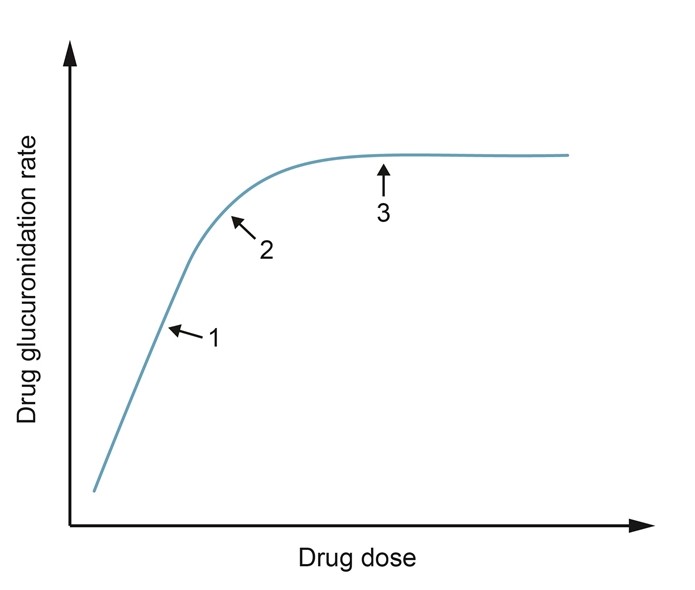 Which of the following is the most accurate statement about this drug's metabolism?
Which of the following is the most accurate statement about this drug's metabolism?
A) A constant proportion of the drug is metabolized past point 3
B) Bioavailability of the drug is highest at point 1
C) Biotransformation of the drug ceases near point 2
D) Metabolism begins to switch to zero-order kinetics near point 2
E) The rate of drug metabolism is not dependent on dose before point 1
Correct Answer:
Verified
Q363: A group of investigators examined the effects
Q364: A 65-year-old man is brought to the
Q365: A group of investigators is studying the
Q366: A 34-year-old kidney transplant patient treated with
Q367: A 68-year-old man comes to the office
Q369: A 16-year-old girl is brought to the
Q370: A 78-year-old man was found to have
Q371: A 57-year-old Caucasian male with severe pyelonephritis
Q372: A clinical trial is being conducted to
Q373: Three alpha-agonist drugs are tested as potential
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents