Saline solutions (NaCl in water) used to deliver intravenous drugs are 0.89%(w/v) . What mass of NaCl would be needed to prepare 450.0 mL of such a solution?
A) 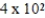 g
g
B) 0.89 g
C) 4.0 g
D) 5.1 g
Correct Answer:
Verified
Q2: When you have your blood drawn, the
Q18: If you have 9 g of NaCl
Q20: Consider the following graph. Q20: The osmotic pressure of a 0.10 M Q23: A solution is made by dissolving 5.84 Q24: What is the molar mass of ibuprofen, Q25: How many grams of solid KCl are Q26: A solution contains 35.5 mg of Vitamin Q26: Calculate the number of moles of ZnCl2, Q29: What is the total molarity of solute![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents