The two compounds below have the same molecular formula. One would expect A to have a higher boiling point than B. 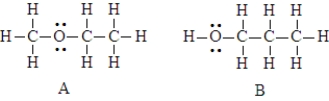
Correct Answer:
Verified
Q1: Consider a bowling ball with the same
Q3: Below is the structure of the amino
Q4: Specific heat is the conversion factor that
Q6: The specific heat of ice is 0.480
Q7: Either the Kelvin or the Celsius temperature
Q9: The following is a mathematical expression of
Q11: The law of conservation of energy states
Q12: Butane is a gas at room temperature
Q15: The pressure of a gas is dependent
Q18: One would expect a compound with the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents