The two compounds below have the same molecular formula. One would expect A to stronger dispersion forces than B. 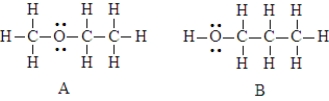
Correct Answer:
Verified
Q2: Which of the following increases as temperature
Q7: Either the Kelvin or the Celsius temperature
Q9: The following is a mathematical expression of
Q11: The law of conservation of energy states
Q12: In order to convert a pressure in
Q16: Oxygen, nitrogen and helium are present in
Q17: To convert from joules to kilojoules, move
Q18: One would expect a compound with the
Q19: Which of the following is true as
Q20: Diethyl ether historically was used as an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents