The compound represented below contains two single covalent bonds. 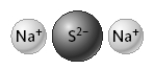
Correct Answer:
Verified
Q1: Potassium tends to form a cation (K+).
Q4: The element Co (cobalt) satisfies the octet
Q9: The formula that corresponds to the IUPAC
Q12: A possible bonding pattern for an element
Q13: Sodium loses an electron in forming an
Q17: Chloride ion (Cl-) is an anion because
Q19: Strontium forms an anion by losing two
Q20: In the following compound, phosphorus (P) would
Q63: Metals gain electrons to become ions in
Q90: The name for the compound formed between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents