The correct formula of an ionic compound containing Fe2+ and ClO 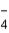 is
is
A) FeClO4
B) Fe2(ClO4) 3
C) Fe(ClO4) 2
D) Fe3(ClO4) 2
Correct Answer:
Verified
Q28: When an ionic compound forms between aluminum
Q32: Which of the following occurs when an
Q36: Calcium carbonate is used as a phosphate
Q38: Which of the following statements is generally
Q38: Using most common charges, the ionic compound
Q43: How many bonding electron pairs are represented
Q44: What is the name of the species
Q52: What is the mass of 1.00 mol
Q53: An ion of phosphorus has a -3
Q54: What is the formula for a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents