Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme involved in cysteine homeostasis, and its overexpression has been linked to asthma, cancer, and other diseases. It catalyzes the addition of water across a bond to cleave extracellular glutathione (GSH) into glutamate and a dipeptide composed of cysteine and glycine. It is believed to function by the mechanism shown in Figure 1.
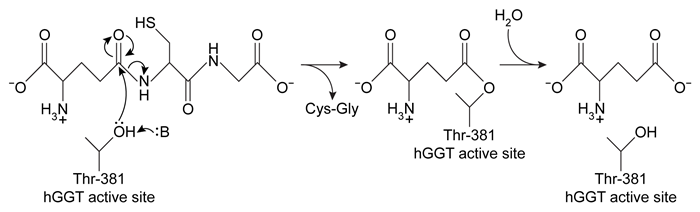 Figure 1 Proposed mechanism of hGGT-mediated GSH cleavageResearchers interested in investigating hGGT inhibitors as a treatment for pathologies related to excess hGGT activity performed the following experiments, each containing the same concentration of hGGT.Experiment 1hGGT was incubated with various concentrations of GSH in the presence or absence of 250-μM candidate compound acivicin or Compound 1. However, both candidates were found to be unsuitable as acivicin showed significant neurotoxicity and Compound 1 acted as an activator instead of an inhibitor.
Figure 1 Proposed mechanism of hGGT-mediated GSH cleavageResearchers interested in investigating hGGT inhibitors as a treatment for pathologies related to excess hGGT activity performed the following experiments, each containing the same concentration of hGGT.Experiment 1hGGT was incubated with various concentrations of GSH in the presence or absence of 250-μM candidate compound acivicin or Compound 1. However, both candidates were found to be unsuitable as acivicin showed significant neurotoxicity and Compound 1 acted as an activator instead of an inhibitor.
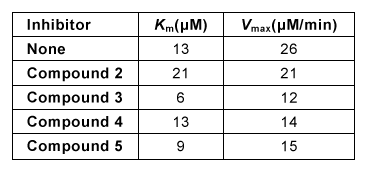
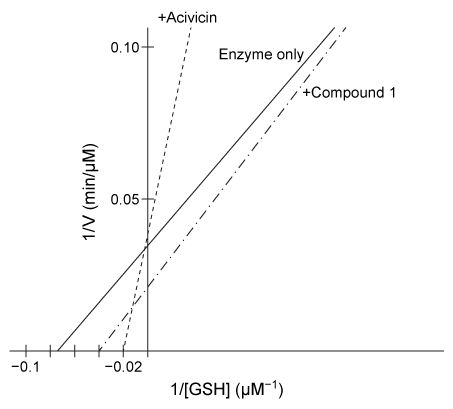 Figure 2 Lineweaver-Burk plot of hGGT activity with respect to GSH in the presence or absence of 250-μM candidate inhibitorsExperiment 2Researchers hypothesized that slight variations in the structure of Compound 1 could result in hGGT inhibition and tested several derivatives, shown in Table 1.Table 1 Kinetic Parameters of hGGT in the Presence or Absence of Several Candidate Inhibitors at 250 μM
Figure 2 Lineweaver-Burk plot of hGGT activity with respect to GSH in the presence or absence of 250-μM candidate inhibitorsExperiment 2Researchers hypothesized that slight variations in the structure of Compound 1 could result in hGGT inhibition and tested several derivatives, shown in Table 1.Table 1 Kinetic Parameters of hGGT in the Presence or Absence of Several Candidate Inhibitors at 250 μM
 Experiment 3To better understand the physiological conditions under which hGGT operates, researchers exposed a cultured cell line expressing hGGT on the cell surface to a solution that mimicked the composition of extracellular fluid, including levels of GSH. They found that in this setup, uninhibited hGGT results in a reaction rate that is approximately ½Vmax.
Experiment 3To better understand the physiological conditions under which hGGT operates, researchers exposed a cultured cell line expressing hGGT on the cell surface to a solution that mimicked the composition of extracellular fluid, including levels of GSH. They found that in this setup, uninhibited hGGT results in a reaction rate that is approximately ½Vmax.
Adapted from Wickham S, Regan N, West MB, et al. Inhibition of human ?-glutamyl transpeptidase: development of more potent, physiologically relevant, uncompetitive inhibitors. Biochem J. 2013;450(3) :547-57.
-Based on Figure 2, Compound 1 activates hGGT in which of the following ways?
A) It increases the turnover number.
B) It decreases the Michaelis constant.
C) It increases the kcat/Km ratio.
D) It decreases the maximum velocity.
Correct Answer:
Verified
Q98: Passage
Synthesis of coenzyme A (CoA) requires vitamin
Q99: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Q100: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Q101: Passage
ATP synthase, also known as Complex V,
Q102: Passage
A newborn girl is diagnosed with a
Q104: Passage
ATP synthase, also known as Complex V,
Q105: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q106: Passage
ATP synthase, also known as Complex V,
Q107: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q108: Atpenins are succinate-ubiquinone reductase inhibitors with antifungal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents