Passage
NDPK is an enzyme in budding yeast that uses ATP to phosphorylate nucleotide diphosphates (NDPs and dNDPs) , yielding the triphosphate form of each (NTPs and dNTPs) . dNTPs are necessary for DNA synthesis, yet yeast cells that lack NDPK grow and divide at the same rate as wild-type yeast. Therefore, triphosphate generation is most likely carried out by one or more other enzymes.The enzyme TMPK converts deoxythymidine monophosphate (dTMP) to the diphosphate form (dTDP) by transferring the γ-phosphate of ATP to dTMP. Yeast cells that lack TMPK activity cannot synthesize DNA and become arrested during replication. It was proposed that TMPK may partially compensate for NDPK loss by phosphorylating dTDP as well as dTMP.To better understand the role of TMPK in nucleotide phosphorylation, wild-type TMPK and the inactive E75K variant were purified by affinity chromatography, and the following experiments were performed.Experiment 1After purification, a sample containing 3 μg of protein was added to a reaction mixture containing radiolabeled nucleotide monophosphates (NMPs or dNMPs) and unlabeled ATP. The reaction proceeded for 30 minutes, and products were separated by thin-layer chromatography (TLC) on a PEI-cellulose plate. PEI-cellulose separates nucleotides by charge (Figure 1) , and migration distance decreases with increasing negative charge. Spots were visualized by autoradiography, and it was determined that 0.30 nmol of dTMP and 0.15 nmol of uridine monophosphate (UMP) became phosphorylated, respectively.
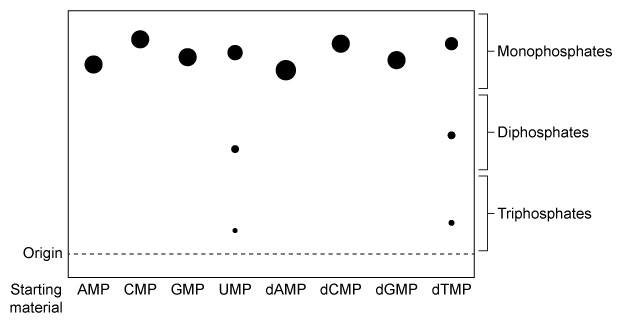 Figure 1 TLC analysis of nucleotide monophosphates after exposure to TMPK and ATP. (Note: Different nucleotides with the same phosphorylation level migrate different distances.) Experiment 2The experiment was repeated using radiolabeled dTDP as the starting material. The reaction proceeded in buffer only, in the presence of E75K TMPK, or in the presence of wild-type TMPK. Products were separated by TLC and visualized (Figure 2) .
Figure 1 TLC analysis of nucleotide monophosphates after exposure to TMPK and ATP. (Note: Different nucleotides with the same phosphorylation level migrate different distances.) Experiment 2The experiment was repeated using radiolabeled dTDP as the starting material. The reaction proceeded in buffer only, in the presence of E75K TMPK, or in the presence of wild-type TMPK. Products were separated by TLC and visualized (Figure 2) .
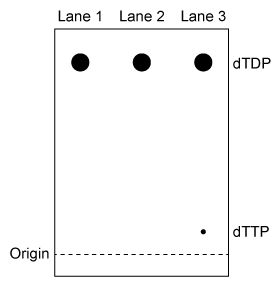 Figure 2 TLC analysis of dTDP phosphorylation with buffer only (Lane 1) , E75K TMPK (Lane 2) , or wild-type TMPK (Lane 3)
Figure 2 TLC analysis of dTDP phosphorylation with buffer only (Lane 1) , E75K TMPK (Lane 2) , or wild-type TMPK (Lane 3)
Adapted from Chien CY, Chen BR, Chou CK, Sclafani RA, Su JY. The yeast Cdc8 exhibits both deoxythymidine monophosphate and diphosphate kinase activities. FEBS Lett. 2009;583(13) :2281-6.
-A Mg2+ ion in the active site of TMPK is directly involved in catalysis. Given this, Mg2+ most likely plays which of the following roles in TMPK-mediated reactions?
A) It binds negatively charged amino acids, stabilizing the tertiary structure of TMPK.
B) It interacts with water molecules in the active site, facilitating phosphate hydrolysis.
C) It binds anions, preventing them from competing with phosphate groups for the active site.
D) It interacts with the negative charges of phosphate groups, stabilizing the transition state.
Correct Answer:
Verified
Q241: Passage
NDPK is an enzyme in budding yeast
Q242: During prolonged fasting, which of the following
Q243: Passage
Many cephalopods can change color to blend
Q244: The reaction between UTP and glucose-1-phosphate (G1P)
Q245: The amino acid methionine can be converted
Q247: Passage
Many cephalopods can change color to blend
Q248: Proteins A, B, C, and D are
Q249: An enzyme was assayed in the absence
Q250: Fatty acid synthesis proceeds according to the
Q251: Passage
NDPK is an enzyme in budding yeast
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents