Passage
Destabilase, an enzyme found in the saliva of leeches, exhibits at least two activities: isopeptidase activity and glycosidase activity. Isopeptidase activity is believed to prevent blood clotting by hydrolyzing the isopeptide bond between the ε-amino group of lysine and the γ-carboxyl group of glutamate in fibrin monomers. The enzyme's glycosidase activity digests sugars found in the peptidoglycans of bacterial cell walls and may play a role in innate immunity.Two isoforms of destabilase were tagged with a polyhistidine tail and recombinantly expressed in E. coli cells. Both isoforms were then purified by affinity chromatography and eluted by imidazole, which competes with histidine-tagged proteins for column binding. Each isoform eluted efficiently with 300 mM imidazole. Five microliters of each purified enzyme were then provided with saturating levels of substrate and assessed for isopeptidase and glycosidase activity at various pH levels, which allowed the optimal pH of enzymatic activities for each isoform to be identified (Figure 1) . Further studies showed that at optimal pH, both isoforms had approximately the same kcat values, but isoform 2 had a lower Km than isoform 1 for both enzymatic activities.
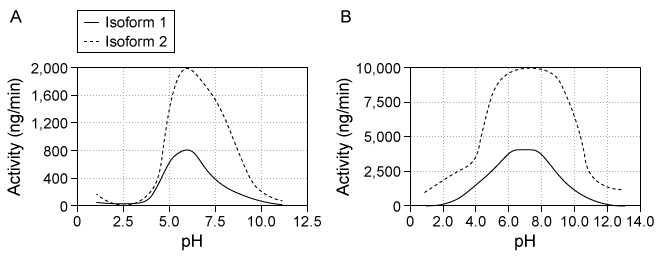 Figure 1 Isopeptidase activity (A) and glycosidase activity (B) of 5 μL of destabilase isoforms 1 and 2 at various pH valuesIsoform 2 was further characterized by mutating specific residues and measuring enzymatic activities at optimal pH. The results are shown in Figure 2.
Figure 1 Isopeptidase activity (A) and glycosidase activity (B) of 5 μL of destabilase isoforms 1 and 2 at various pH valuesIsoform 2 was further characterized by mutating specific residues and measuring enzymatic activities at optimal pH. The results are shown in Figure 2.
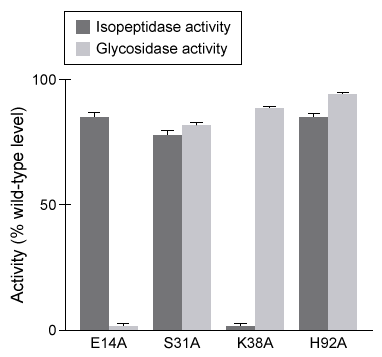 Figure 2 Isopeptidase and glycosidase activity of isoform 2 of destabilase with various mutations
Figure 2 Isopeptidase and glycosidase activity of isoform 2 of destabilase with various mutations
-Which conclusion is LEAST supported by the data in Figure 2?
A) Glutamate at position 14 is required for glycosidase activity but not isopeptidase activity.
B) Mutating serine at position 31 has no effect on isopeptidase activity or glycosidase activity.
C) Lysine at position 38 is required for isopeptidase activity but not glycosidase activity.
D) Mutating histidine at position 92 has the smallest impact on glycosidase activity.
Correct Answer:
Verified
Q276: Passage
Porphyrin biosynthesis is a multistep process that
Q277: Passage
Whole grains have been shown to reduce
Q278: Passage
Whole grains have been shown to reduce
Q279: Passage
Whole grains have been shown to reduce
Q280: Passage
The Ankyrin repeat and SOCS box containing
Q282: Passage
Destabilase, an enzyme found in the saliva
Q283: Passage
Oxidative stress occurs in cells when reactive
Q284: Passage
Oxidative stress occurs in cells when reactive
Q285: Passage
Oxidative stress occurs in cells when reactive
Q286: Researchers quantified the amount of protein produced
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents