Passage
The nicotinic acetylcholine receptor (nAChR) is a protein embedded in the muscle fiber membrane that facilitates cell-to-cell communication across the neuromuscular junction. When acetylcholine (ACh) binds nAChR, electrical depolarization within skeletal muscle fibers is induced and ultimately gives rise to contractile activity. The nAChR is composed of five protein subunits symmetrically organized around a central ion pore. Previous studies demonstrated that these subunits may serve as epitopes (antibody binding sites) in autoimmune disease. By reversibly binding nAChR subunits, anti-nAChr antibodies may temporarily block the attachment site for ACh, resulting in muscle weakness.Researchers harvested muscle samples from mice to investigate the effects of anti-nAChR antibodies on muscle tension, the pulling force transmitted by muscles during contraction.Experiment 1First, skeletal muscle samples (tibialis anterior) of adult male mice were surgically harvested (n = 30) , washed, and stripped of connective tissues. Next, the isolated muscle samples were placed in a 150-mL oxygen-infused, electrolyte-rich bath containing glucose and Ca2+ ions that were able to enter the muscle fibers and enable maximal contractile force. One end of the submerged muscle was anchored in place while the other was attached to an apparatus that measures muscle tension and modifies muscle length. Using electrodes, action potentials were elicited in the harvested muscle tissue to evaluate contractile function (Figure 1) .
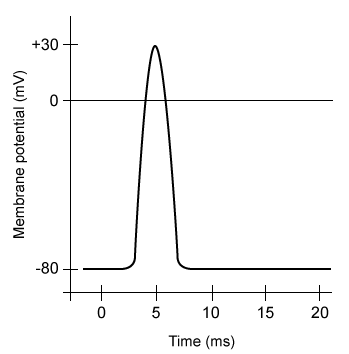 Figure 1 Action potential elicited from a harvested skeletal muscle sampleExperiment 2The researchers divided the 30 muscle samples into three groups of 10. Resting muscle tension was measured, and the electrolyte-rich baths of each group were further infused with a 7.5-mL solution containing mouse-specific anti-nAChR antibodies, ACh, or a mixture of both (Table 1) . Post-infusion (peak) muscle tension was measured for 30 minutes, with results displayed in Figure 2.Table 1 Solutions Infused into the Electrolyte-Rich Bath
Figure 1 Action potential elicited from a harvested skeletal muscle sampleExperiment 2The researchers divided the 30 muscle samples into three groups of 10. Resting muscle tension was measured, and the electrolyte-rich baths of each group were further infused with a 7.5-mL solution containing mouse-specific anti-nAChR antibodies, ACh, or a mixture of both (Table 1) . Post-infusion (peak) muscle tension was measured for 30 minutes, with results displayed in Figure 2.Table 1 Solutions Infused into the Electrolyte-Rich Bath
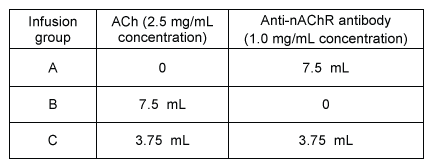
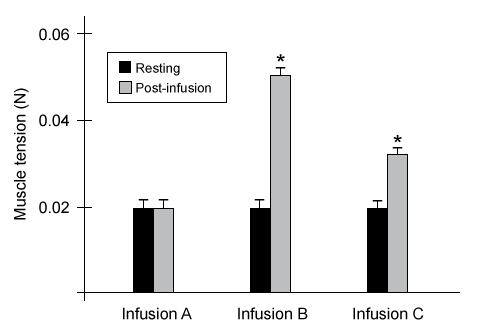 Figure 2 Pre-infusion resting muscle tension and post-infusion peak muscle tension in harvested muscle samples (Note: * indicates p < 0.05.)
Figure 2 Pre-infusion resting muscle tension and post-infusion peak muscle tension in harvested muscle samples (Note: * indicates p < 0.05.)
-Why was Ca2+ added to the oxygen-infused, electrolyte-rich bath? To allow Ca2+ to:
A) be sequestered inside the sarcoplasmic reticulum of the muscle fibers.
B) enter the mitochondria to promote sufficient ATP production by muscle fibers.
C) increase the affinity of muscle fiber myoglobin for oxygen.
D) facilitate the binding of ACh to its receptor on the muscle fiber.
Correct Answer:
Verified
Q248: Researchers are screening bacterial chromosomes for mutations
Q249: Researchers are studying a single-celled pathogen. Which
Q250: Given that a species diverged from its
Q251: One function of catecholamines released by the
Q252: The North American flying squirrel and the
Q254: Passage
The nicotinic acetylcholine receptor (nAChR) is a
Q255: Passage
The nicotinic acetylcholine receptor (nAChR) is a
Q256: Plasmodium falciparum is a protozoan parasite known
Q257: In neurons, action potential propagation occurs along
Q258: Passage
The sex chromosomes X and Y are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents