Passage
Gastric acid is one of many acids important to biological function in the human body. Production of the main component of gastric acid, hydrochloric acid (HCl) , is accomplished in the stomach by the parietal cells, as shown in Figure 1.
 Figure 1 Formation of gastric acid in the stomachFacilitated by the carbonate dehydratase enzyme (1) , the parietal cells utilize the interaction between aqueous carbon dioxide and water in the blood as a source of bicarbonate ions and acidic H+ ions (protons) . The H+ ions produced in this process are then exchanged for potassium ions through the cell membrane by a proton pump, the H+/K+-ATPase (2) . In conjunction with this proton exchange, bicarbonate ions are also exchanged for chloride ions from the interstitial fluid. As a result, H+ and Cl− ions are concentrated in the lumen of the stomach to form aqueous hydrochloric acid.Because the production of the acid proceeds with a drop in pH from 7.0 in the cell cytosol to 1.0 in the stomach lumen, the process occurs against a concentration gradient and is endergonic, requiring the input of energy supplied by the hydrolysis of ATP. Ignoring effects from ion charge, the free energy change for ion transport across the cell membrane (ΔGt) can be estimated by Equation 1:
Figure 1 Formation of gastric acid in the stomachFacilitated by the carbonate dehydratase enzyme (1) , the parietal cells utilize the interaction between aqueous carbon dioxide and water in the blood as a source of bicarbonate ions and acidic H+ ions (protons) . The H+ ions produced in this process are then exchanged for potassium ions through the cell membrane by a proton pump, the H+/K+-ATPase (2) . In conjunction with this proton exchange, bicarbonate ions are also exchanged for chloride ions from the interstitial fluid. As a result, H+ and Cl− ions are concentrated in the lumen of the stomach to form aqueous hydrochloric acid.Because the production of the acid proceeds with a drop in pH from 7.0 in the cell cytosol to 1.0 in the stomach lumen, the process occurs against a concentration gradient and is endergonic, requiring the input of energy supplied by the hydrolysis of ATP. Ignoring effects from ion charge, the free energy change for ion transport across the cell membrane (ΔGt) can be estimated by Equation 1:
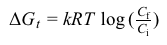 ∆Gt=kRT logCfCiEquation 1where R is the gas constant (8.32 J/mol·K) , k is a logarithm conversion constant, T is the absolute temperature, and Ci and Cf are the initial and final molar concentrations across the gradient, respectively.Although dilute, the gastric (hydrochloric) acid released into the lumen yields a strongly acidic environment crucial to protein digestion and the destruction of harmful micro-organisms. A comparison of its acid dissociation constant (Ka) to those of other biologically relevant organic acids is given in Table 1.Table 1 Comparison of Strengths of Selected 0.10 M Acids at 25°C
∆Gt=kRT logCfCiEquation 1where R is the gas constant (8.32 J/mol·K) , k is a logarithm conversion constant, T is the absolute temperature, and Ci and Cf are the initial and final molar concentrations across the gradient, respectively.Although dilute, the gastric (hydrochloric) acid released into the lumen yields a strongly acidic environment crucial to protein digestion and the destruction of harmful micro-organisms. A comparison of its acid dissociation constant (Ka) to those of other biologically relevant organic acids is given in Table 1.Table 1 Comparison of Strengths of Selected 0.10 M Acids at 25°C
 Adapted from Koolman J, Roehm KH. Color Atlas of Biochemistry. Thieme; 2011: 268-271; and Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. Macmillan; 2005: 397-398, 419, 520.
Adapted from Koolman J, Roehm KH. Color Atlas of Biochemistry. Thieme; 2011: 268-271; and Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. Macmillan; 2005: 397-398, 419, 520.
-Based on the biological system described in the passage, how many millimoles of hydrogen ions are present in 10.0 mL of gastric acid?
A) 100 mmol
B) 10 mmol
C) 1.0 mmol
D) 0.10 mmol
Correct Answer:
Verified
Q116: Passage
Although acetylsalicylic acid (aspirin) is a well-known
Q117: The Nernst equation states: Q118: Passage Q119: Passage Q120: Passage Q122: Passage Q123: Passage Q124: Which of the following is a combustion Q125: Passage Q126: Passage![]()
Although acetylsalicylic acid (aspirin) is a well-known
Although acetylsalicylic acid (aspirin) is a well-known
A group of students were given water
Potassium permanganate (KMnO4) is a highly reactive
A group of students were given water
Gastric acid is one of many acids
Gastric acid is one of many acids
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents