Passage
The alkaline-earth metals are a highly reactive family of metals occupying Group 2 (IIA) of the periodic table. In their elemental form, the alkaline-earth metals have a shiny, silvery-white to slightly yellowish appearance. The alkaline-earth metals exhibit some similarities to the chemistry of the alkali metals (Group 1) but are a little less reactive.Atoms of the alkaline-earth metals (M) have only two valence electrons and a low first and second ionization energy. Consequently, these metals readily lose both electrons to produce cations with a +2 charge (M+2) during reactions with nonmetals. As a result, the alkaline-earth metals do not exist in their elemental metallic form in nature. Instead, the Group 2 elements are found primarily as metal oxides, which form by reacting oxygen (Reaction 1) .
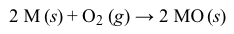
Similarly, elemental samples of alkaline-earth metals will react with elemental halogens (X2) to form metal halides (Reaction 2) , all of which are ionic except for BeCl2.
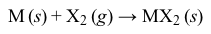
Except for beryllium, the alkaline-earth metals react with water to produce very basic metal hydroxide solutions and hydrogen gas (Reaction 3) .
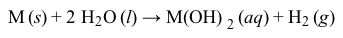
The most difficult alkaline-earth metal to study is radium, because it is a rare radioactive element that is produced from the nuclear decay of uranium. The most common natural isotope, radium-226, has a half-life of 1,600 years and decays by alpha emission into radon-222 (Reaction 4) .
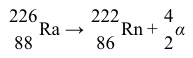
-Based on the reaction in Figure 1 and the energy profile in Figure 2, which statement correctly describes both Compound 2 and Compound 3?
A) Compound 2 and Compound 3 are both the kinetic products of the reaction.
B) Compound 2 is the kinetic product, and Compound 3 is the thermodynamic product.
C) Compound 2 is the thermodynamic product, and Compound 3 is the kinetic product.
D) Compound 2 and Compound 3 are both the thermodynamic products of the reaction.
Correct Answer:
Verified
Q138: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q139: Considering the type of element represented by
Q140: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q141: An element that exists in its standard
Q142: Passage
The alkali metals are a highly reactive
Q144: Passage
The alkali metals are a highly reactive
Q145: Passage
The alkaline-earth metals are a highly reactive
Q146: Passage
The alkaline-earth metals are a highly reactive
Q147: Passage
The alkaline-earth metals are a highly reactive
Q148: Passage
The alkaline-earth metals are a highly reactive
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents