The graph below shows the redox titration curve of a 0.05 M Sn2+ solution titrated with 0.1 M Ce4+. 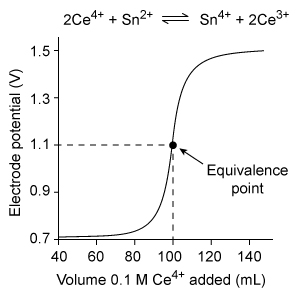 The equivalence point indicates the volume of 0.1 M Ce4+ required to fully oxidize all of the Sn2+ in solution. If more than 100 mL of 0.1 M Ce4+ is required to titrate an equal volume of another 0.05 M Sn2+ solution, which of the following could be used to explain the inaccurate equivalence point?
The equivalence point indicates the volume of 0.1 M Ce4+ required to fully oxidize all of the Sn2+ in solution. If more than 100 mL of 0.1 M Ce4+ is required to titrate an equal volume of another 0.05 M Sn2+ solution, which of the following could be used to explain the inaccurate equivalence point?
A) The volume of the Sn2+ solution has increased.
B) The concentration of Sn2+ in solution has increased.
C) An impurity that can oxidize Ce3+ back to Ce4+ is present in the Sn2+ solution.
D) A metal salt impurity that can also be oxidized by Ce4+ is present in the Sn2+ solution.
Correct Answer:
Verified
Q204: Passage
Copper plays a vital role as a
Q205: Passage
Hyperbaric oxygenation therapy involves placing a patient
Q206: Passage
Hyperbaric oxygenation therapy involves placing a patient
Q207: Under anaerobic conditions, hemoglobin (HbFe2+) can react
Q208: Passage
Copper plays a vital role as a
Q210: The reactivity of three oxides mixed in
Q211: The diagram below shows the activity of
Q212: Passage
Copper plays a vital role as a
Q213: Passage
Hyperbaric oxygenation therapy involves placing a patient
Q214: Passage
Hyperbaric oxygenation therapy involves placing a patient
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents