An unknown metal M forms an ionic hydroxide with the formula M(OH) 2 that exhibits the equilibrium 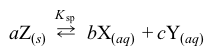 aqin a saturated aqueous solution. If the solution pH is 10, the solubility product constant Ksp of the compound is:
aqin a saturated aqueous solution. If the solution pH is 10, the solubility product constant Ksp of the compound is:
A) 5.0 × 10−31.
B) 5.0 × 10−13.
C) 1.0 × 10−12.
D) 1.0 × 10−8.
Correct Answer:
Verified
Q293: Which of the following equations correctly represents
Q294: Passage
Oxytocin is a naturally occurring peptide hormone
Q295: During beta decay, a radioactive atomic nucleus
Q296: Passage
Oxytocin is a naturally occurring peptide hormone
Q297: Which of the following will occur during
Q299: Consider the equilibrium that exists for a
Q300: Passage
Oxytocin is a naturally occurring peptide hormone
Q301: The density of ethanol varies slightly with
Q302: What are the products of the alpha
Q303: A reaction between chloride and permanganate ions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents