Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3) 3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3) 2. 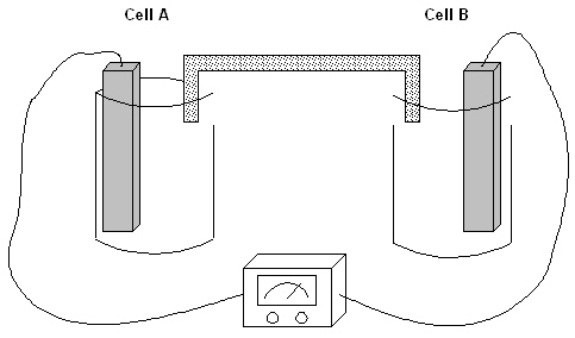
Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?
A) 3Fe(NO3) 2(aq) + 2Al(NO3) 3(aq) + 6e- → 2Al(s) + 3Fe(s) + 5NO3-(aq)
B) 3Fe(s) + 2Al3+ → 2Al(s) + 3Fe2+
C) Fe(s) + Al3+ → Al(s) + Fe3+
D) Al(s) + 2Fe3+ → 2Fe(s) + 3Al3+ (aq)
E) None of the above
Correct Answer:
Verified
Q6: Exhibit 17-1
The following question(s) pertain to the
Q7: Exhibit 17-1
The following question(s) pertain to the
Q8: Exhibit 17-1
The following question(s) pertain to the
Q9: Exhibit 17-1
The following question(s) pertain to the
Q10: Exhibit 17-1
The following question(s) pertain to the
Q12: Exhibit 17-1
The following question(s) pertain to the
Q13: Exhibit 17-2
The following question(s) pertain to an
Q14: Exhibit 17-2
The following question(s) pertain to an
Q15: Exhibit 17-2
The following question(s) pertain to an
Q16: Exhibit 17-2
The following question(s) pertain to an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents