Which of the following is the titration curve of weak base being titrated by a strong acid
A) 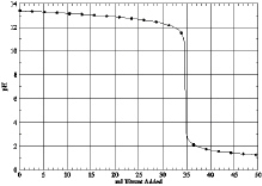
B) 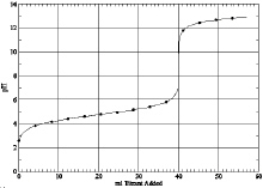
C) 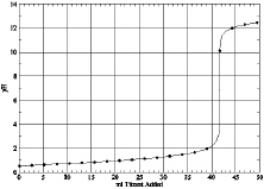
D) 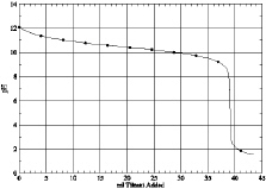
Correct Answer:
Verified
Q3: The value of Kw increases slightly over
Q4: Which of the following is the strongest
Q5: In pure water:
A) There are no detectible
Q6: Ka = 6.6×10-4 for the reaction:
HF
Q7: Which of the following is the titration
Q9: Exhibit 15-1
The following question(s) pertain to the
Q10: Exhibit 15-1
The following question(s) pertain to the
Q11: A student has 100.0 ml of 0.65
Q12: A student has 50.0 ml of 0.30
Q13: A student mixes 60.0 ml of 0.30
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents