Given the phase diagram below, at 200K and a pressure of 150 atm. What phase(s) is (are) present? 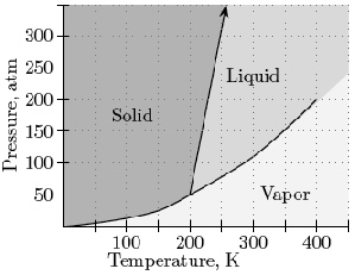
A) gas
B) liquid
C) solid
D) liquid and solid
E) gas and liquid
Correct Answer:
Verified
Q15: Which of the following is true for
Q16: A supercritical fluid has a density that
A)
Q17: At the triple point
A) solid, liquid, and
Q18: For the following phase diagram the area
Q19: The process of a solid converting directly
Q21: The triple point of a compound is
Q22: For a given substance, the vapor pressure
Q23: Which do you think will have the
Q24: Which do you think has a higher
Q25: In the phase diagram of water increasing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents