A Lewis dot structure for benzene, C6H6 would give two equivalent resonance structures for this cyclic molecule with alternating single and double bonds 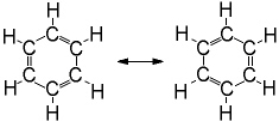 A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A) 3s(C2p-C2p) bonds between each C atom
B) 2s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
C) 1s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
D) 1s(Csp2-Csp2) bonds between each C atom, and 2p MO delocalized over the C's
E) 1s(Csp2-Csp2) bonds between each C atom, and 3p MO delocalized over the C's
Correct Answer:
Verified
Q17: What are the VB wavefunctions for the
Q18: What do you predict for the hybridization
Q19: In the molecule, H-CºN, the VB model
Q20: In the molecule, H-CºN, the VB model
Q21: In the LCAO wavefunction for H2
Q23: VB theory is poorly suited to polar
Q24: The Born Openheimer approximation assumes that the
Q25: The lowest energy antibonding orbital in H2+
A)
Q26: The ionization energy of H2- would be
Q27: A MO orbital for a heteronuclear
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents